北京佰司特科技有限责任公司
7 年
手机商铺
- NaN
- 0.5
- 0.5
- 1.5
- 0.5
推荐产品
公司新闻/正文
多类器官串联芯片培养技术
3299 人阅读发布时间:2024-04-29 19:43
翻译整理:北京佰司特科技有限责任公司
德国 TissUse 公司目前主要专注于多器官串联芯片的设备和芯片生产,同时也提供相关的技术方案和试剂,
所以是目前可以做「Multi-Organ-Chip」和「Human-on-a-chip」的方案。

一、类器官的技术与原理介绍:
类器官,具有某一器官多种功能性细胞和组织形态结构的三维(3D)培养物,主要来源于人具有多项分化潜能的多能干细胞(包括人胚胎干细胞和人诱导多能干细胞 iPSCs)或成体干细胞。人多能干细胞能分化为个体所有类型的细胞,在体外,经过诱导分化,模拟人体器官发育过程,能使人多能干细胞直接分化形成各种类器官;不同组织器官都存在内源组织干细胞,在维持各器官的功能形态发挥着重要作用。这些干细胞在体外一定的诱导条件下,可以自组织形成一个直径仅为几毫米的具有组织结构和多种功能细胞的三维培养物。器官芯片是获取两个或两个以上不同的类器官,并且放置在特定的培养芯片上进行共培养,能模拟人体的多个器官参与的生理学过程。
与传统 2D 细胞培养模式相比,3D 培养的类器官包含多种细胞类型,能够形成具有功能的「微器官」,能更好地用于模拟器官组织的发生过程及生理病理状态,因而在基础研究以及临床诊疗方面具有广阔的应用前景。
科学家 Lancaster 和 Knoblich,这样定义类器官:
器官特异性细胞的集合。这些细胞从干细胞或器官祖细胞发育而来,并能以与体内相似的方式经细胞分序(cell sorting out)和空间限制性的系别分化而实现自我组建」。
基于这一定义,可以发现类器官具备这样几个特征:
1.必须包含一种以上与来源器官相同的细胞类型;
3.细胞的组织方式应当与来源器官相似。
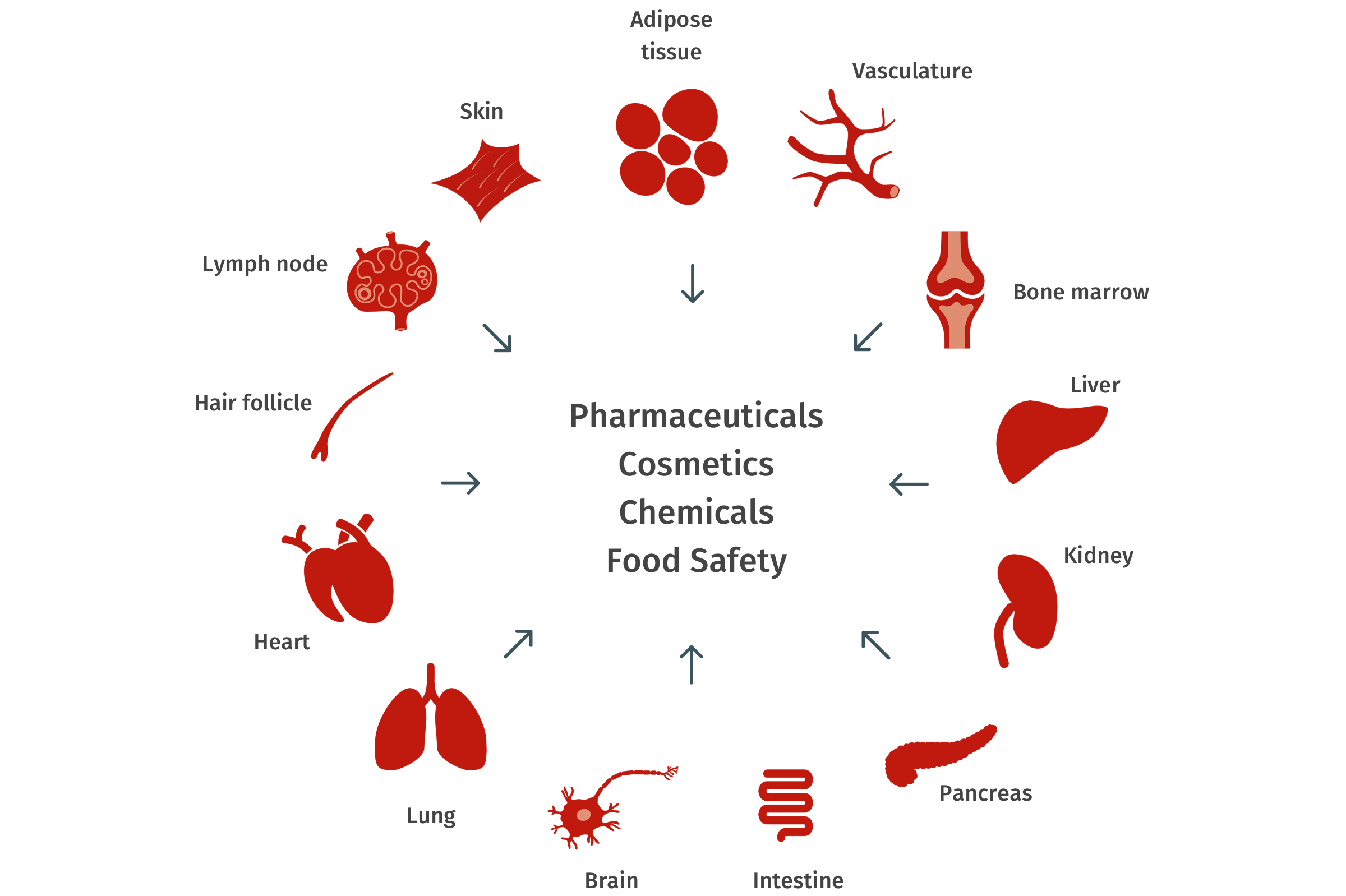
二、类器官在医疗领域的应用案例
类器官在复受损的人类肝脏中的作用
2021 年 2 月发表在 Science 杂志上的一篇研究报告中报道,来自英国剑桥大学等机构的科学家们首次揭示胆管类器官可以修复受损的人类肝脏。文章中,他们利用一种技术在实验室中培育出胆管类器官(bile duct organoids),即胆管微型器官,同时他们发现,这种胆管类器官可用于修复受损的人类肝脏。这是这种技术首次用于人体器官。
研究人员所开发的这种新方法能利用最近的「灌注系统」将捐赠的器官在体外进行维持。利用这种技术,他们首次证实可以将实验室中培养的胆管细胞移植到受损的人类肝脏中进行修复,作为方法的原则性证明,他们修复了由于胆管损伤而被认为不适合移植的肝脏,这种方法可能能够应用于多种器官和疾病,以加速细胞治疗的临床应用。
类器官在产生血细胞治疗骨髓相关疾病中的作用
同一个月,美国辛辛那提儿童医院等机构的科学家们在 Nature 杂志上刊文表示,他们成功构建了骨髓图谱,或为利用血液类器官产生血细胞开辟了新的道路。文章中,研究人员使用多种细胞分析技术构建出了骨髓组织「图谱」,相关研究发现或能帮助研究人员理解微小血管如何对骨髓进行组织化及调节血液。研究者认为,这种血液类器官或能用来产生具有特定遗传变异的血细胞群体,而这些血细胞或能被用来开发改善疾病的新型疗法。
类器官在研究消化系统对神经性疾病的影响中的作用
2021 年 1 月份,发表在国际杂志 Science Advance 上的一篇研究报告中,来自麻省理工学院等机构的科学家们通过进行类器官的研究来揭示消化系统对神经性疾病的影响;文章中,研究人员将大脑和循环免疫细胞添加到他们的多器官系统中。他们将帕金森氏病患者的成纤维细胞转化为多能干细胞,随后将其诱导分化为不同类型的脑细胞-神经元,星形胶质细胞和小胶质细胞,研究者将其用于帕金森氏症模型的细胞携带一种突变,该突变会导致一种称为 α 突触核蛋白的蛋白质蓄积,从而损害神经元并引起脑细胞炎症。
类器官在肺细胞命运与疾病研究中的作用
2021 年 12 月发表在 Cell Press 上的一篇综述表示,Derek C. Liberti 和 Edward E. Morrisey 在综述中概述了类器官的新研究方法以进一步探索肺生物学中的新概念。研究分析类器官与体内建模相结合的益处,以探索呼吸系统中的各种生态位和隔室如何对急性和慢性肺部疾病作出反应。类器官技术的战略实施和改进将为理解和确定改善肺部疾病状态的新治疗方法提供令人振奋的新机会。除了科学家们在类器官研究领域取得的多项重要研究成果外,关于类器官领域的支持性政策也在相继推出,类器官能用于建立疾病的相关模型,与 2D 疾病模型相比,其在阐明疾病的发展、稳态和发病机制方面更具优势。
类器官在 SARS-CoV-2 感染导致肾脏纤维化研究中的作用
2021 年 12 月 25 日,德国 Jansen, J 团队在 Cell Stem Cell 报告称,SARS-CoV-2 可以直接感染肾细胞,与患者尸检样本中的肾小管间质纤维化增生有关。研究中用 SARS-CoV-2 感染了人源性多能干细胞衍生的肾脏类器官。结果,随着亲纤维化信号通路的激活,类器官中细胞出现了损伤,并检测到胶原蛋白 I 的表达增加。而 SARS-CoV-2 蛋白酶抑制剂能够减少胶原蛋白 I 的表达并改善细胞损伤。这些数据解释了新冠感染患者出现急性肾损伤的原因,也证明了类器官是研究病毒感染优秀的体外模型。
类器官在研究常见卵巢癌的起源中的作用
2021 年 12 月 29 日发表在 Cell Reports 杂志上的一篇研究中,来自洛杉矶 Cedars-Sinai 医疗中心的研究者们报告,他们利用 BRCA1 突变的 hipsc 衍生的输卵管类器官模型重现了早期癌的发生。研究表明,具有 BRCA1 突变的输卵管类器官为个性化机制和药物筛选研究提供了一个平台,并为携带 BRCA1 突变的女性提供了个性化早期检测和预防策略的基础。
类器官在建立 hiPSC 衍生皮肤类器官模型研究 SARS-CoV-2 感染中的作用
2021 年 12 月 31 日,发布在 Advanced Science 上的一项研究报道,Jie Ma 等人培养了一种由 hiPSC 衍生的具有毛囊和神经系统的皮肤类器官来研究其对 SARS-CoV-2 感染的易感性。结果表明,COVID-19 可以直接影响皮肤中的毛囊和神经,这为 COVID-19 与脱发之间的关系提供了证据。且其也能为后续研究 SARS-CoV-2 感染机制和受其影响的皮肤患者的药物筛查提供指导。此外,本研究中构建的皮肤类器官包括皮肤的表面和深层结构,这也使其可能成为其他皮肤病研究的理想模型。
三、类器官技术现在的发展形势
2021 年,类器官被列为「十四五」国家重点研发计划重点专项,随着相关研究不断深入,我国类器官技术水平将进一步提升,同时在相关企业积极布局下,类器官市场化程度将不断提升。有关资料显示,2020 年全球类器官市场规模在 5 亿美元左右,随着医疗技术进步,类器官市场规模将进一步扩张,预计 2021-2026 年,全球类器官市场规模将保持以 18.2% 的年均复合增长率增长。全球范围内,类器官市场主要集中在北美、欧洲等地区,其中北美地区类器官市场增速高于全球平均水平。近日,国家药品监督管理局药品审评中心(CDE)连发三个与基因治疗、细胞治疗相关的指导原则,首次将类器官列入基因治疗及针对基因修饰细胞治疗产品的验证指南当中。
2021 年,来自国家科学技术部的文件,「基于干细胞的人类重大难治性疾病模型」被被列为「十四五」首批重点专项,文件明确指出,恶性肿瘤是长期严重影响我国人民健康的重大难治性疾病之一,而类器官作为重要的新兴前沿技术,能够为疾病研究与治疗助力。由于类器官可以模拟体内真实器官的三维结构与功能,为精准医疗提供了全新的研究方法和治疗手段,在一系列生物学与生物医学中都有着广阔的应用前景,我们有理由期待未来类器官将在生物学及临床医学研究领域发挥越来越大的作用。
①万方数据库检索结果:纳入 187 篇文献,2019 年类器官中文研究呈爆发式增长,其中肠类器官模型研究较多,主要应用领域包括精准医疗、肿瘤研究和个体化医疗;②Web of Science 数据库 (核心集) 检索结果:共纳入 2 450 篇文献,采用 Histcite 软件分析 20 篇高被引文章筛选出 5 条类器官原始经典文献,分别介绍了肠道类器官、多能干细胞衍生的三维大脑类器官、从活检标本和循环肿瘤细胞中长期培养的前列腺癌类器官、含有肾单位的肾类器官及人胃组织三维类器官模型的构建方法,均分别为各领域的开山之作,为类器官后续研究打下了基础;③临床注册信息检索结果:检索到与类器官研究有关的中国临床注册方案 13 篇,北美临床注册方案 23 篇,美国类器官临床研究领域更广泛,开展时间更早,但多为队列研究和单臂试验,而中国近年来在类器官临床研究领域已经取得了一些成果,有 2 个受人瞩目的随机对照试验方案正在进行中;④SooPAT 中国专利数据库检索结果:共检索到 55 项有权专利,主要专利技术涉及 3D 大脑类器官新型培养方法,高通量 3D 细胞、类组织及类器官动态培养系统的研发等;⑤上述数据说明,目前研究者已经能够成功构建出多种模拟人体器官功能的类器官体外模型 (如肠、脑、肾及各种癌组织),但由于缺乏足够的临床随机对照试验证据,因此临床适用性还有待探讨,而利用癌症患者的肿瘤组织直接培养生成体外肿瘤类器官,分析潜在的药物治疗靶点,进行抗癌药物筛选及开发抗肿瘤新药,将是未来类器官领域主要研究方向。
作为一种新型的药物筛选和药敏检测模型,类器官应用在近两年来初露锋芒。作为一种体外模型,与传统的单层细胞培养模型相比,类器官从形态、功能、基因表达、药物作用等方面与体内环境更加接近,药物筛选结果也更贴近体内实验结果。在疾病研究、肿瘤药敏、临床免疫、药物毒理、再生医学等多学科领域有重要的应用,具有广阔的发展前景及极大的应用潜力。

四、类器官技术的政策法规
根据欧洲委员会(EC)提交给理事会和欧洲议会(EC,2013d)的报告,2011 年,欧盟(EU)成员国有 1150 万只动物被用于实验和其他科学目的。报告指出,用于人类医学、牙科和兽医学研究和开发的动物数量自 2008 年的上一份报告以来已从 22.8% 下降到 18.8%。用于毒理学和其他安全性评价的动物数量占总数的 8.75%,相对保持不变。然而,用于基础生物学研究的动物比例急剧上升,从 38% 上升到 46%。到目前为止,这三个领域在欧盟用于科学目的的动物数量最多(2011 年为 870 万)(Daneshianetal.,2015)。利用动物进行的药物分子的生物学研究所获得的信息是制药业和生物技术新药物发展的基础。欧盟报告强调了这一事实,眼研究,骨代谢,生育能力研究,效能测试,免疫原性测试,神经学和免疫学的研究领域,肿瘤的病理生理机制研究,用于疾病治疗目的获得药物作用机制等等是基础生物学研究需求增加的主要原因。但是,过去几十年的研究和发展已经清楚地表明,来自动物研究的数据往往不能很好地反映人类的状况(HackamandRedelmeier,2006;LeistandHartung,2013;Matthews,2008;Olsonetal.,2000;Pereletal.,2007;HartungandLeist,2008;Schnerchetal.,2010;Senaetal.,2010;Seoketal.,2013;vanderWorpetal.,2010;Hartung,2013)。这个结论可以从药物在临床试验中的反复失败中看出。临床试验各个阶段的候选物成功率的行业基准分别为:一期 48-64%,二期 29-32%,三期 60-67%(Cooketal.,2014;Hayetal.,2014)。因此,这里提出的报告侧重于药物测试难题的药物开发方面。新药开发目前面临两大障碍(Rovidaetal.,2015a):目前的药物开发方法在临床试验之前甚至在临床试验期间都有很高的候选药物消耗,不断增加的有关临床前测试的监管要求下,为避免对人体的伤害导致的研发效率降低(Fig.1)。过去七年的数据标明药物开发成本的显著增加(美国医药研究与制造商协会,PhRMA),而美国食品和药物管理局(FDA)新药批准率波动是由于药物开发而引起的事故导致的。研发生产率已经下降了超过 15 倍,通胀调整后的平均支出分别从 20 世纪 70 年代的每一种成功药物(包括失败的成本)的 1.79 亿美元增加到 21 世纪的 26 亿美元(Scannelletal.,2012;TuftsCSDD,2014)。
基于微生理系统的人的单器官和多器官工程,在此基础上建立的测试系统有望模拟不同的疾病阶段,并在临床试验之前评估毒性、免疫原性、ADME 谱和治疗效果。这一技术将对药物发展的未来产生重大影响。此外,基于微生理系统的分析可能会彻底改变我们当前的模式,对任何新物质(例如用于农业、食品、生态系统或化妆品)的危害进行排序,从而取代目前使用的实验动物模型。
几乎所有的监管机构都鼓励使用器官芯片的数据作为药品 IND 申报的材料:
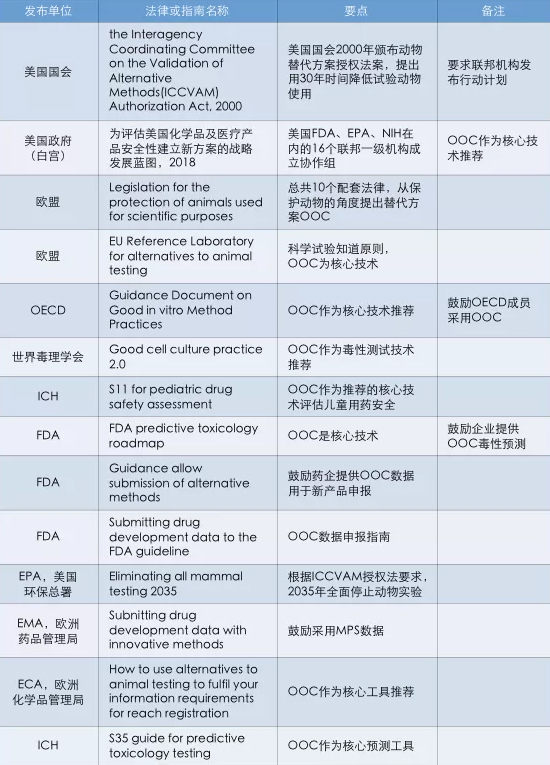
CDE(国家药品监督管理局药品审评中心)首次将类器官列入基因治疗和细胞治疗非临床研究与评价推荐模型
2021 年 12 月 3 日,CDE(国家药品监督管理局药品审评中心)发布三条与基因治疗和细胞治疗相关的指导原则,首次将类器官列入基因治疗及针对基因修饰细胞治疗产品的指导原则当中。
具体如下:
①《基因治疗产品非临床研究与评价技术指导原则(试行)》指出:
一、基因治疗产品药理学研究「如果没有合适的动物模型满足试验需要,应当依据科学原理开发相应的动物模型或使用更完善的体外试验系统、替代性模型(例如类器官)开展试验。」
二、由于种属和免疫状态的差异,基因治疗产品在人体内的表达、分布和作用在模型动物中可能有较大不同,可选用替代产品(如基因修饰的模型动物细胞 、组织和类器官等)进行 POC 研究。
②《基因修饰细胞治疗产品非临床研究技术指导原则(试行)》指出:
可采用基于细胞和组织的模型(如类器官等)为预测基因修饰细胞在患者人群中的有效性和安全性评估提供有用的补充信息。
与此同时,众多企业也开始加速涌入这一赛道,投入巨额资金以尽快促进该技术实现商业化落地。目前主要的市场参与者包括 TissUse、CN Bio、Emulate、InSphero、Mimetas、Ascendance 等。国内相对有小幅落后,以学术机构的探索为主,另外还有大橡科技、子瞻生物等企业在进行相关研究和应用。
五、德国 TissUse 公司的多类器官串联培养方案:
- TissUse GmbH,2010 年成立,德国柏林
- 起源于柏林工业大学
- 专注于类器官培养系统研究,FDA 推荐的技术方案
- 30 名研究员 +22 名其他员工
- 9 项专利族 +117 项专利
目前已有众多组织在主动推动器官芯片的相关研究。FDA 与 Emulate/TissUse 等几家公司签订合作协议,将器官芯片技术作为毒理学测试平台,这证明该技术在新药筛选上的作用得到美国方面的认可;美国国立卫生研究院 (NIH) 运营的「药物筛选组织芯片」项目,旨在资助与微芯片设备相关的研究;美国国防高级研究计划局则将此类设备用于国家安全研究;同时,由于器官芯片技术在很大程度上减少了动物在研究中的应用规模,因此动物伦理治疗协会对于器官芯片的开发持大力支持态度;欧盟则向 5 个组织提供了总计 140 万欧元的支持资金,用于器官芯片设备的研发。来自科学家做出了杰出的科学贡献。The 11th world congress on Alternatives and Animal Use in the Life Sciences(第 11 届世界生命科学替代和动物使用大会)邀请 TissUse 的 Dr. Uwe Marx 做了主题讲座 The ultimate alternative to testing in animals and human volunteers(人类微生理系统的科学、伦理和接受——在实验室动物和人类志愿者中进行测试的最终选择)。此外,TissUse 科学家的 Dr. Uwe Marx 获得了 the Humane Society of the United States 颁发的 Russell and Burch Award 2021 奖,表彰 TissUse 器官芯片技术在减少了动物在研究中的使用所做出的贡献。
目前基本上所有的企业都是以生产芯片为主,兼顾技术服务和方案建设,而且都是单个的器官芯片。德国 TissUse 公司目前主要专注于多器官串联芯片的设备和芯片生产,同时也提供相关的技术方案和试剂,所以是目前能做「Multi-Organ-Chip」和」Human-on-a-chip」。
德国 TissUse 公司的多类器官串联培养技术:
德国 TissUse 公司目前主要专注于多器官串联芯片的设备和芯片生产,同时也提供相关的技术方案和试剂,所以是目前能做「Multi-Organ-Chip」和「Human-on-a-chip」。
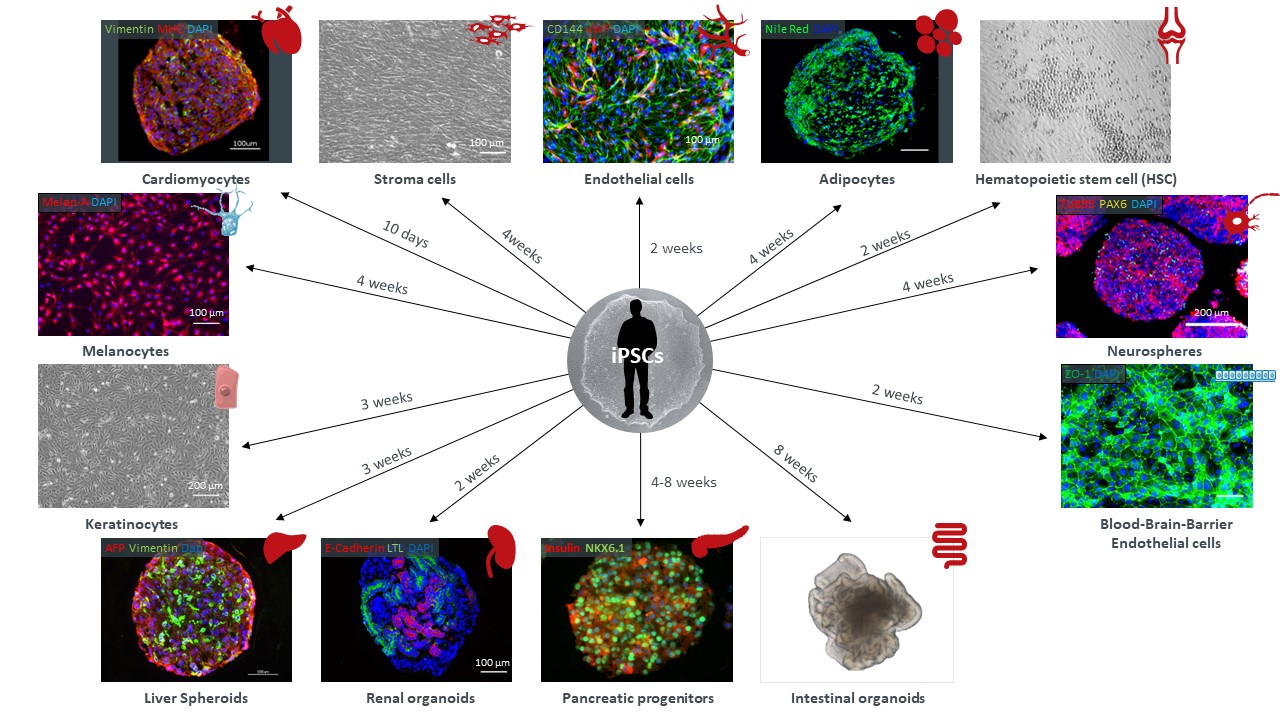
a) 细胞培养及诱导分化技术
通过筛选不同成分的培养基,不同种类的诱导分化因子,不同浓度的诱导分化因子,诱导分化的时间,实现体外诱导 iPS 细胞分化成不同类器官组织。
b) 芯片可控 3D 共培养技术
通过功能化组织器官模块化设计,仿生血管网络建立和可控流体关注,实现 3D 肝脏,胰岛和肾脏组织动态,可控的培养
c) 复合类器官芯片功能模块集成技术
依据体内肝脏,胰岛和肾脏解剖上的独立性和生理功能的相关性,将不同的功能模块单元通过微流控通道网络连接,建立具有时空分辨,灵活操作优点的复合类器官芯片功能模块集成技术。以肝、胰岛组织为基础,建立肝-胰复合器官功能兼容的新技术,对共培养体系中流体灌注条件,营养成分筛选,培养条件优化,实现两种组织的动态共培养。并通过糖稳态反馈调控验证其功能偶联。以肝、肾岛组织为基础,建立肝-肾复合器官功能兼容的新技术,对共培养体系中流体灌注条件,营养成分筛选,培养条件优化,实现两种组织的动态共培养以及各自生理功能的最大化,通过嵌铂药物的代谢和排泄来验证肝-肾的生理相关性。
d) 类器官复合芯片关键功能验证
肝-胰复合类器官芯片糖异常病理模拟及功能验证:面向疾病研究和药物评价的需求,以链脲霉素(STZ)诱导胰岛 b 细胞损伤构建胰岛素缺乏引起的糖平衡调节的病理状态,然后应用降糖药物在芯片系统进行模拟治疗和药物评价。通过监测复合芯片中胰岛组织的糖响应功能以及肝类器官糖利用情况,研究药物在人体器官系统中的降糖效应,观察肝组织对药物的代谢效应以及药物代谢导致的药效改变规律。上述研究将通过模拟体内糖代谢过程中重要实质性脏器协调作用,为临床治疗提供新的策略思路。
肝-肾复合类器官芯片药物代谢模拟及功能验证:对降糖药物的肝、肾毒性进行评估,同时考察药物代谢影响肝,肾毒性的规律及机理。包括对肝细胞活性,白蛋白分泌,尿素合成进行监测,对肝细胞代谢酶 CYP3A4,CYP2C9,CYP1A2 相关蛋白的表达进行检测,肾组织的屏障完整性,滤过功能,跨膜电阻等的检测。利用液质联用对药物的动态变化进行分析,解析药物的代谢成分,观察肝对药物的代谢效应以及药物代谢导致的药物肾毒性作用及机制。
同时筛选 10 种以上候选药物,利用肝-胰,肝-肾复合器官芯片进行药物代谢,药效,毒性评价,为干细胞来源的复合类器官的药物评价提供可靠的理论和数据支持。
六、德国 TissUse 公司的多类器官串联培养方案
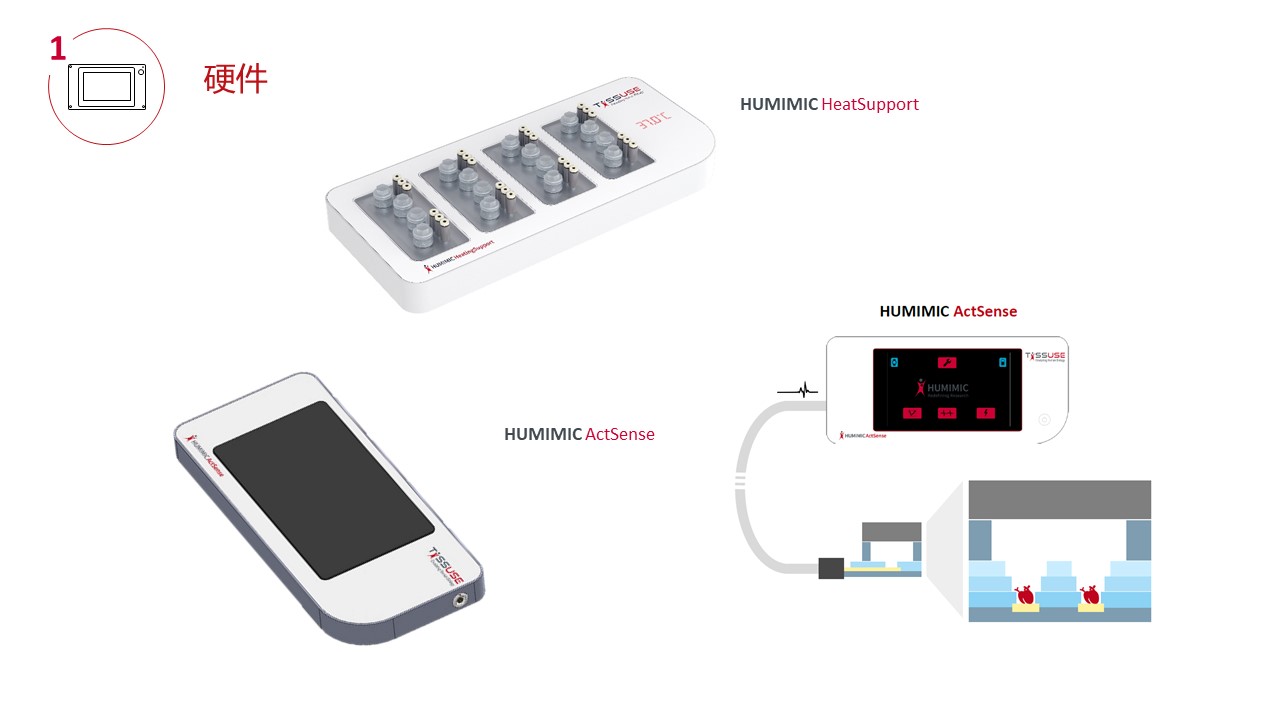
一、专业化的硬件(控制单元)
主机(控制单元)是一个紧凑的台式设备,能够模拟人体内生理环境,包括温度、压力、真空度、微流道循环频率、时间等参数。
7寸触摸显示器,控制面板可以在整个过程中对每个多器官芯片分别进行调节,无需外接电脑,软件操控友好;
可以自主设置每个器官芯片的培养条件,包括温度、压力、真空度、微流道循环频率、时间等参数;
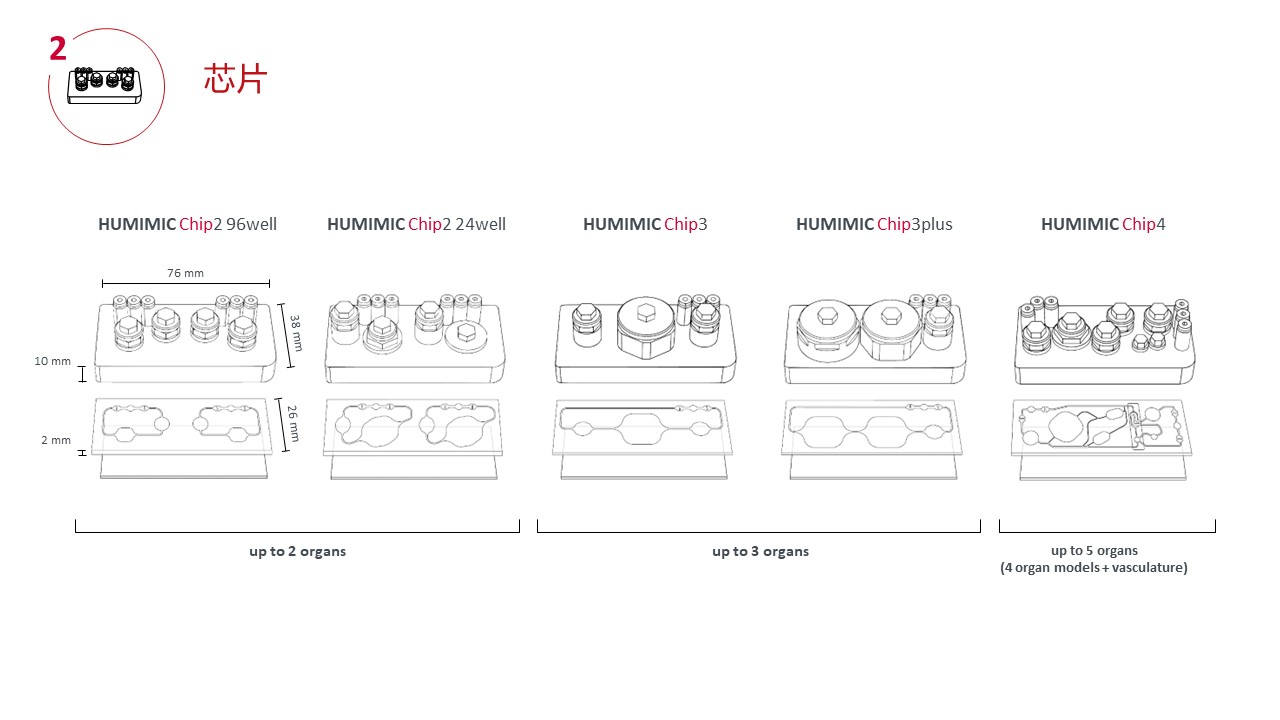
可串联培养2个不同(或相同)、3个不同的、4个不同的类器官;
3个连接拓展口,用于连接其他设备;
同时操控高达8个Chip3 / Chip3 plus,4个Chip2 /Chip4或这些的组合;

二、类器官芯片
芯片有不同的微流道设计,针对不同的器官可以单独设置提供相应的培养条件,提供精准的培养和分化环境;
芯片的泵腔内的柔性膜通过连接的管道,受到压力或真空的作用,在微流道之中产生脉动体流;
二联类器官芯片可以在一个芯片上串联培养2个不同(或相同)的类器官;
三联类器官芯片可以在一个芯片上串联培养3个不同的类器官;
四联类器官芯片可以在一个芯片上串联培养4个不同的类器官;
三、服务方案(细胞、试剂,诱导方案)


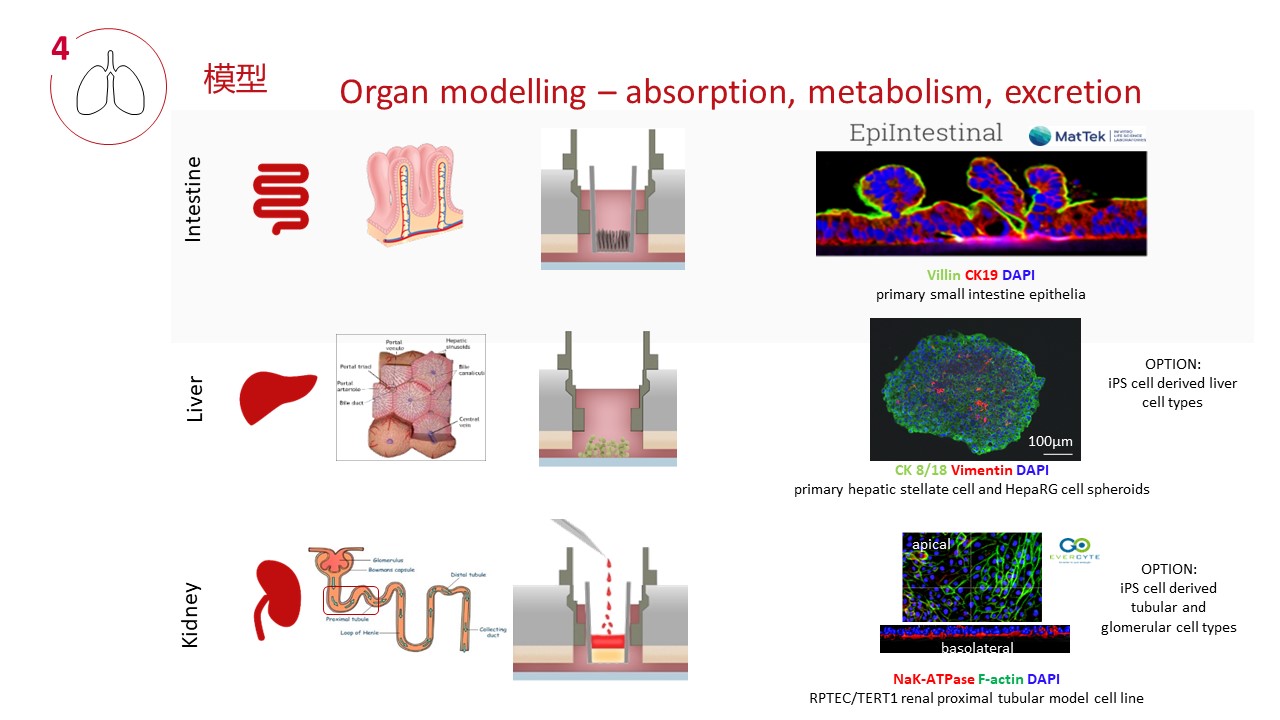
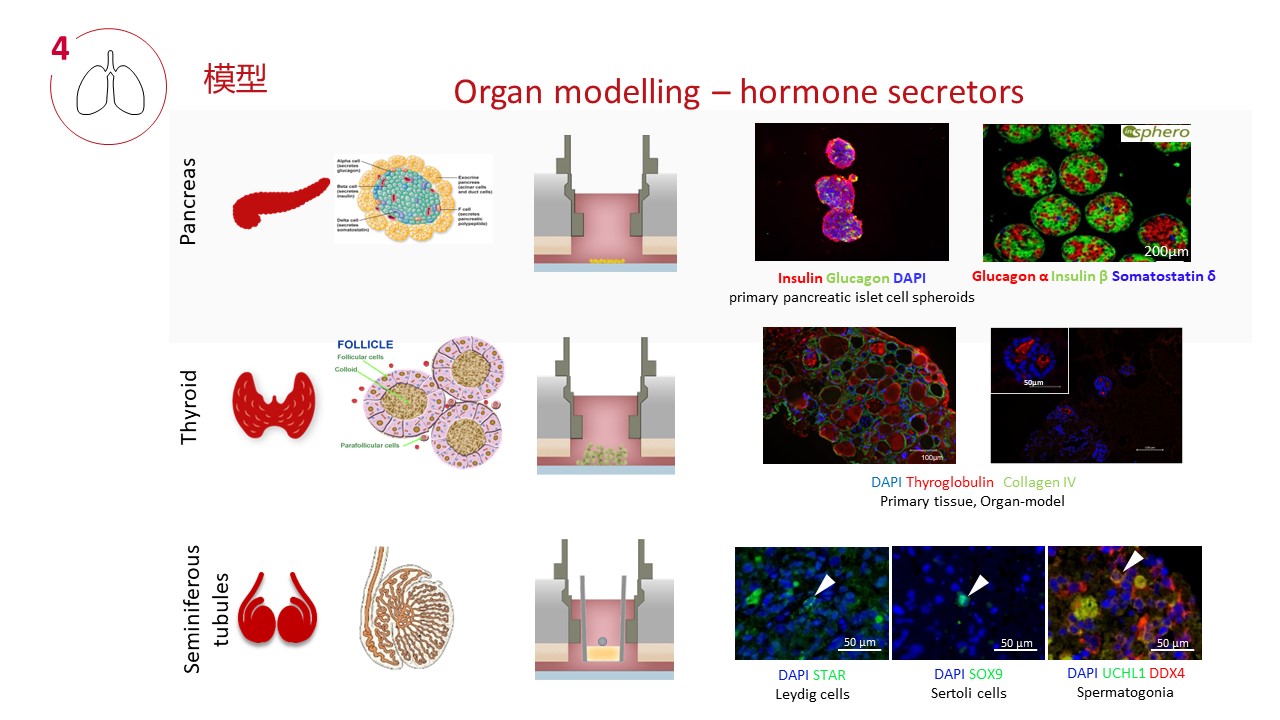
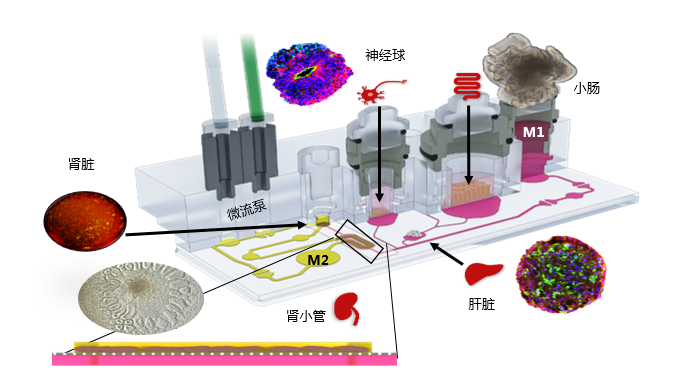
四、器官模型和串联培养技术


类器官串联培养系统---HUMIMIC的应用案例
1、神经球和肝脏的串联共培养(柏林工业大学)-二联器官共培养的药物敏感性
2015, Journal of Biotechnology,
A multi-organ chip co-culture of neurospheres and liver equivalents for long-term substance testing
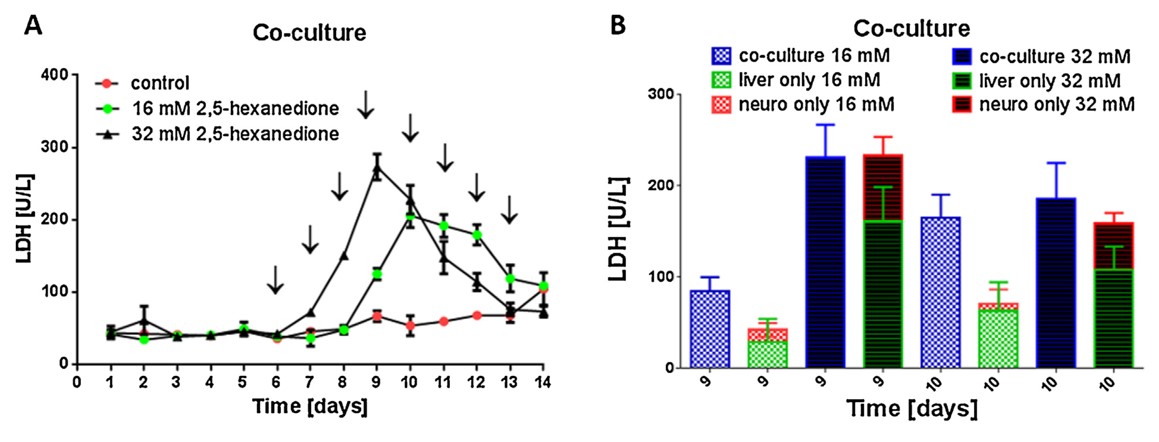
目前用于药物开发的体外实验平台无法模拟人体器官的复杂性,而人类和实验室动物的系统差异巨大,因此现有的方案都不能准确预测药物的安全性和有效性。德国、葡萄牙和俄罗斯的研究团队通过TissUse GmbH公司的微流控多器官芯片(MOC)平台,测试毒物对多器官的作用,揭示了基于微流控的多器官串联共培养能够更好的模拟人体的生理学环境。在体外培养条件下,由于氧气和营养供应有限,类器官培养往往会随着时间的推移而去分化。然而微流控系统中通过持续灌注培养基,更好地控制环境条件,如清除分泌物和刺激因子,并且培养基以可控流速通过,以模拟血流产生的生物剪切应力,因此类器官培养物可以保持良好的生长状态。
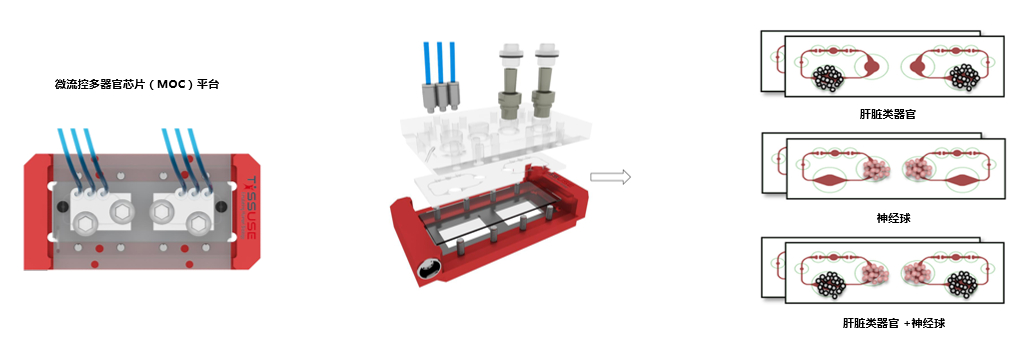
双器官串联芯片(2-OC)能够串联共培养人的神经球(NT2细胞系)和肝脏类器官(肝HepaRG细胞和肝HHSteC细胞)。在持续两周的实验中,反复加入神经毒剂2,5-己二酮,引起神经球和肝脏的细胞凋亡。跟单器官培养相比,串联共培养对毒剂更敏感。因此,多器官串联共培养在临床研究中可以更准确地预测药物的安全性和有效性。推测这是因为一个类器官的凋亡信号导致了第二个类器官对药物反应的增强,这一推测得到了实验结果的支持,即串联共培养的敏感性增加主要发生在较低浓度药物中。
2、心脏肝脏骨骼皮肤的串联共培养(哥伦比亚大学)-四联器官共培养的复杂通讯模型
哥伦比亚大学的科学家也开发了一种多器官串联芯片,建立了串联共培养心脏、肝脏、骨骼、皮肤的技术,发表于2022年的Nature Biomedical Engineering,中通过血液循环串联培养4个类器官,保持了各个类器官的表型,还研究了常见的抗癌药阿霉素对串联芯片中的类器官以及血管的影响。结果显示药物对串联共培养类器官的影响与临床研究结果非常相似,证明了多器官串联共培养能够成功的模拟人体中的药代动力学和药效学特征。
“最值得注意的是,多器官串联芯片能够准确的预测出阿霉素的心脏毒性和心肌病,这意味着,临床医生可以减少阿霉素的治疗剂量,甚至让患者停止该治疗方案。“
Gordana Vunjak-Novakovic, Department of Biomedical Engineering, Columbia University
3、胰岛和肝脏在芯片上的串联共培养(阿斯利康)-二联器官共培养的反馈通讯
2017, Nature Scientific Reports,
Functional coupling of human pancreatic islets and liver spheroids on-a-chip: Towards a novel human ex vivo type 2 diabetes model
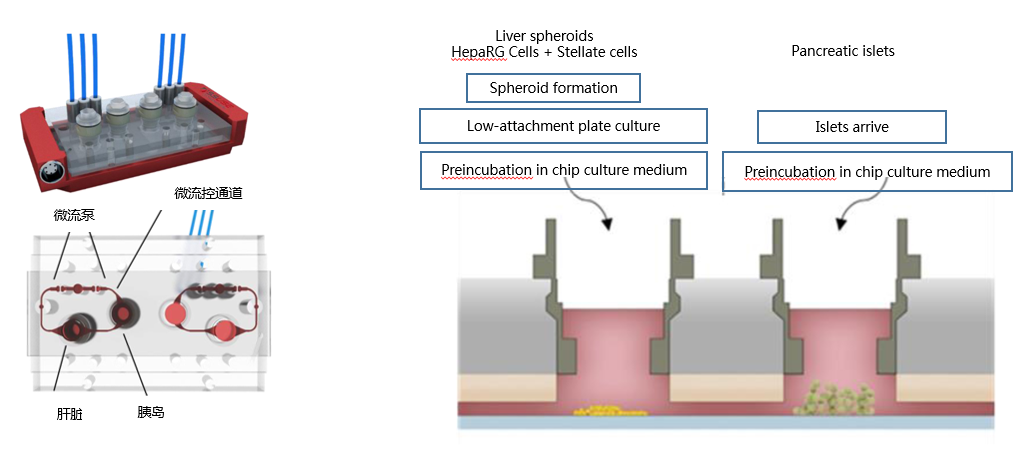
人类系统性疾病的发生过程都是通过破坏两个或多个器官的自我平衡和相互交流。研究疾病和药疗就需要复杂的多器官平台作为体外生理模型的工具,以确定新的药物靶点和治疗方法。2型糖尿病(T2DM)的发病率正在不断上升,并与多器官并发症相关联。由于胰岛素抵抗,胰岛通过增加分泌和增大胰岛体积来满足胰岛素不断增加的需求量。当胰岛无法适应机体要求时,血糖水平就会升高,并出现明显的2型糖尿病。由于胰岛素是肝脏代谢的关键调节因子,可以将生产葡萄糖的平衡转变为有利于葡萄糖的储存,因此胰岛素抵抗会导致糖稳态受损,从而导致2型糖尿病。过去已经报道了多种表征T2DM特征的动物模型,但是,从动物实验进行的研究往临床上转化的效果不佳。更重要的是,目前使用的药物,虽然能缓解糖尿病症状,但对疾病进一步发展的治疗效果有限。
胰腺和肝脏是参与维持葡萄糖稳态的两个关键器官,为了模拟T2DM,阿斯利康(AstraZeneca)的科学家利用TissUse GmbH公司的微流控多器官芯片(MOC)平台,通过微流控通道相互连接,建立一个双器官串联芯片(2-OC)模型,实现芯片上胰腺和肝脏类器官的串联共培养,在体外模拟了胰腺和肝脏之间的交流通讯。
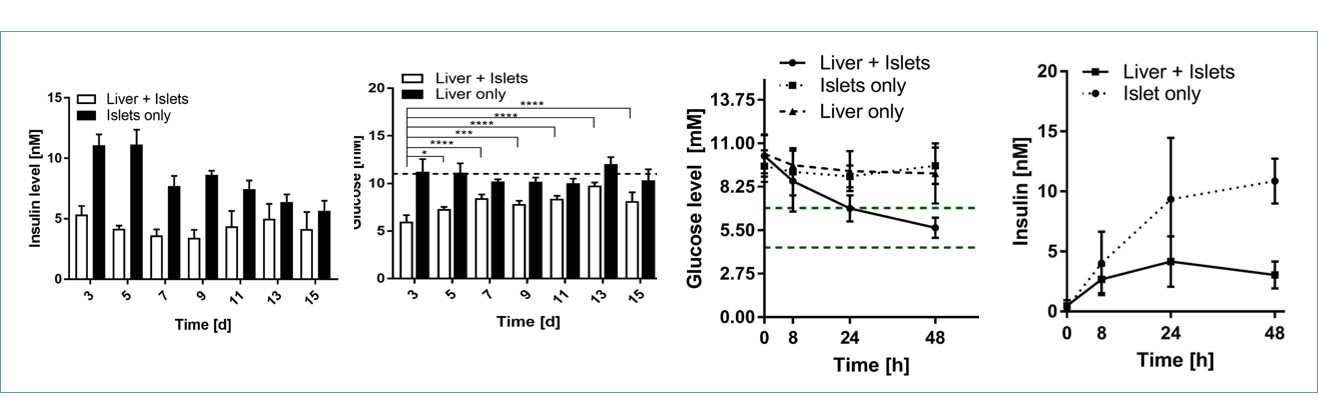
建立串联共培养类器官(胰岛+肝脏)和单独培养类器官(仅胰岛或肝脏),在培养基中连续培养15天,串联共培养显示出稳定、重复、循环的胰岛素水平。而胰岛单独培养的胰岛素水平不稳定,从第3天到第15天,降低了49%。胰岛与肝球体串联共培养中,胰岛可长期维持葡萄糖水平,刺激胰岛素分泌,而单独培养的胰岛,胰岛素分泌显著减少。胰岛分泌的胰岛素促进了肝球体对葡萄糖的利用,显示了串联共培养中类器官之间的功能性交流。在单独培养中的肝球体中,15天内循环葡萄糖浓度稳定维持在~11 mM。而与胰岛共培养时,肝球体的循环葡萄糖在48小时内降低到相当于人正常餐后的水平度,表明胰岛类器官分泌的胰岛素刺激了肝球体摄取葡萄糖。
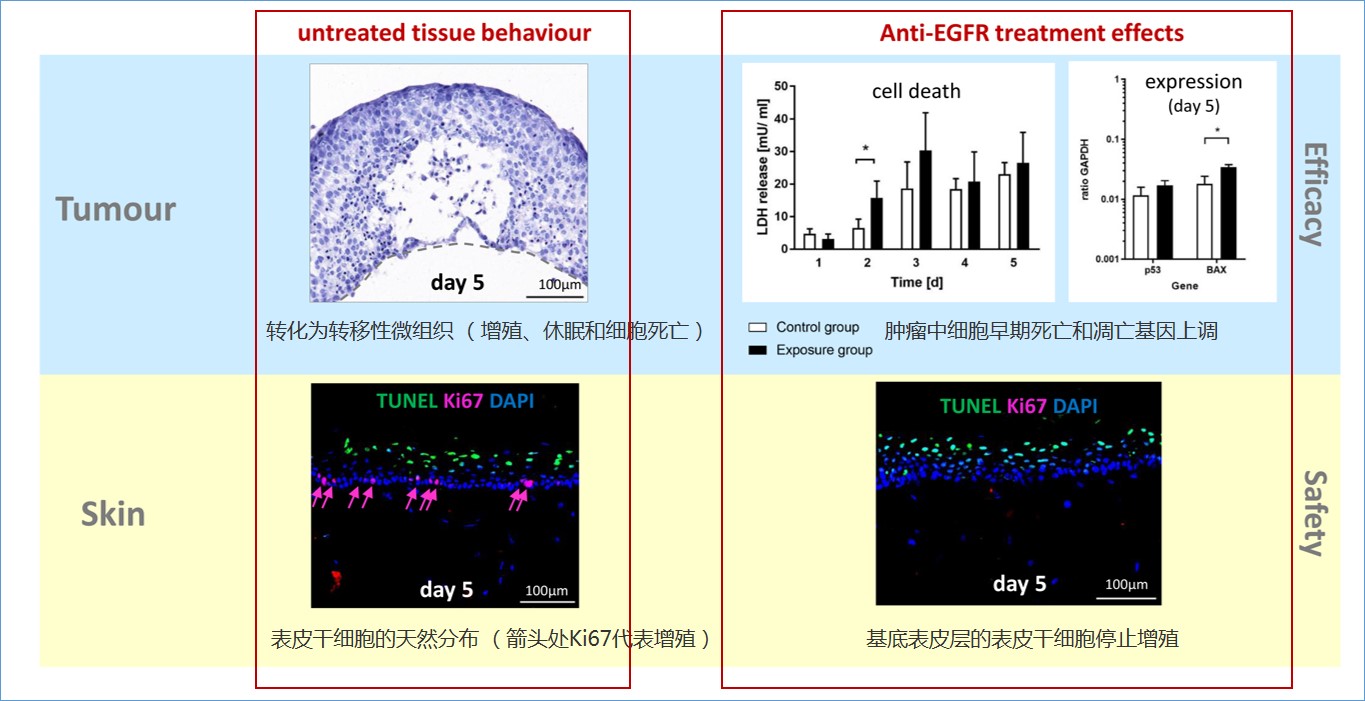
4、肺肿瘤和皮肤在芯片上的串联共培养(拜耳)-抗体药物对肿瘤和正常器官的影响
针对EGFR抗体的药物在癌症治疗中被广泛应用。然而,抗癌药物的使用量与皮肤不良反应成正比相关,皮肤毒性是上皮生长因子受体(EGFR) 靶向治疗中最常见的副作用。但是对于后者的预测目前的方法均无法实现。
双器官串联芯片(2-OC)模型,实现芯片上皮肤和肿瘤的共培养,用于模拟重复给药的剂量实验,同时还生成安全性和有效性的数据,可以在非常早的阶段检测到西妥昔单抗cetuximab对皮肤的几个关键副作用。这种体外分析能够在临床表现之前预评估毒性副作用,可以替代动物试验,有望成为评价EGFR抗体和其他肿瘤药物治疗指数的理想工具。
5、皮肤-肝脏在芯片上的串联共培养(拜尔斯道夫公司)—评估化妆品不同的给药途径
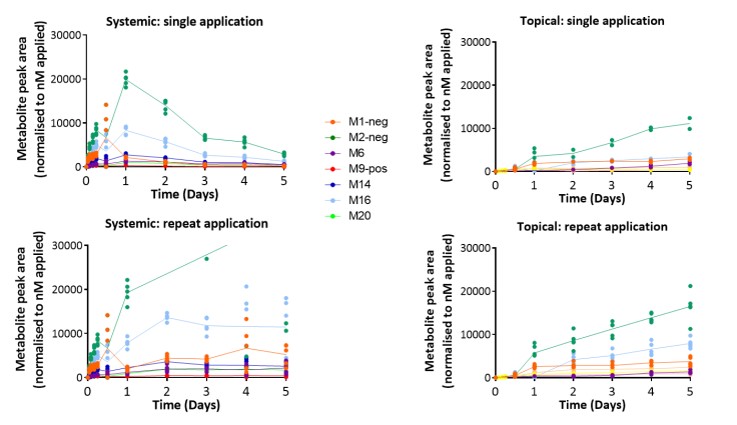
一种独特的基于芯片的组织培养平台已经开发出来,使化妆品和药物对一套微型人体器官的影响测试成为可能。这种“人-片”平台旨在生成可复制的、高质量的人体物质安全性预测体外数据。被测物质进入表皮或在表皮内代谢,然后泵入肝脏并激活相应的CYPs。因此,在肝脏和皮肤的联合培养中,多器官芯片是一种有前途的体外方法,用于全身和局部剂量的化妆品和药物。
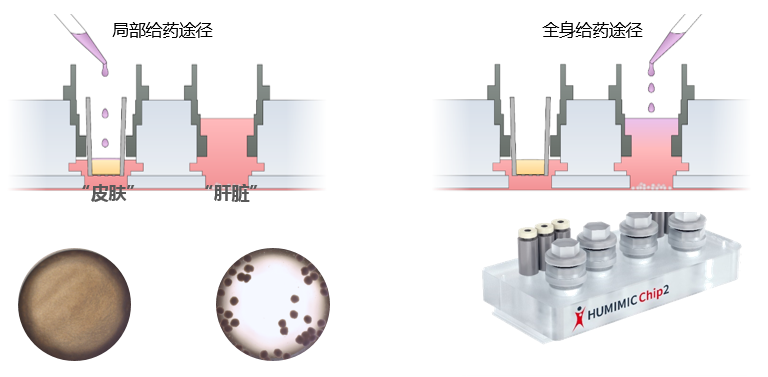
皮肤等效物的培养整合在一个系统中。芯片上的微泵使代谢运输和附加的生理剪切应力成为可能。肝脏和皮肤等效物存活10天,并显示紧密连接和特异性转运蛋白的表达。每天服用咖啡因、维甲酸和倍他米松-21-戊酸,持续7天,以研究已知可被皮肤和肝脏代谢的化合物的作用。将表面敷于表皮的效果与直接敷于培养基的效果进行比较,分析对皮肤渗透和代谢的影响。对肝脏和皮肤等价物进行代谢酶、转运体、分化标记物的表达和活性分析。结果显示,在蛋白水平和mRNA水平上,根据不同物质处理,ⅰ、ⅱ期酶均有本构性和诱导性表达。因此,在肝脏和皮肤的联合培养中,多器官芯片是一种有前途的体外方法,用于全身和局部剂量的药物和化妆品。
6、肺类器官在芯片上的培养(菲莫国际)-空气环境对呼吸道的影响
使用类人肺模型研究吸入气溶胶的沉积和吸附,从而使体外人体呼吸毒性的数据更加准确和可预测。目前的体外气溶胶暴露系统通常不能模拟这些特性,这可能导致在体外生物测试系统中交付非现实的、非人体相关的可吸入试验物质剂量。模拟和研究体外气溶胶暴露装置-吸入器可主动呼吸、操作医用吸入器,或吸吸烟草制品。此外,它可以填充从人类呼吸道不同区域分离的三维上皮细胞。包括口腔、支气管和肺泡细胞培养物的气溶胶传递和相容性的概念的研究,将其应用于测试系统,吸入产生的生理条件下,测试表现在人的呼吸道的方式。这种方法的优点是,它无需花费昂贵、耗时和具有科学挑战性的工作来确定体内提供的剂量,默认情况下,适用于任何测试烟草燃烧产生的气体和任何测试成分。
此外,通过功能和结构上培养人的呼吸道器官模型,该系统消除了在处理呼吸道不同区域时重复暴露与吸烟环境,并能够测试任何相关的呼吸模式或行为。由于该系统能够自行产生或取样测试气溶胶,且其方式与人类呼吸道的做法高度相似,因此消除了在外部测试大气产生或取样过程中引入实验人工制品的风险。
通过建立类器官培养和鉴定平台,培养人肺类器官模型,研究烟草(包括电子烟)燃烧后的气体对人体内健康的影响,从而领导烟草行业的一场技术变革,以创造一个无害烟的未来,并最终以无害烟产品取代香烟,从而造福于那些原本会继续吸烟的成年人、社会、公司。
类器官串联培养系统---HUMIMIC的参考文献
2023, Journal of Applied Toxicology, Early View, Application of a skin and liver Chip2 microphysiological model to investigate the route-dependent toxicokinetics and toxicodynamics of consumer-relevant doses of genistein
Tao TP, Brandmair K, Gerlach S, Przibilla J, Schepky A, Marx U, Hewitt NJ, Maschmeyer I, Kühnl J
2023, Journal of Applied Toxicology, Early View, Suitability of different reconstructed human skin models in the skin and liver Chip2 microphysiological model to investigate the kinetics and first-pass skin metabolism of the hair dye, 4-amino-2-hydroxytoluene
Brandmair K, Tao TP, Gerlach S, Przibilla J, Schepky A, Marx U, Hewitt NJ, Kühnl J, Maschmeyer I
2023, Scientific reports, Vol. 13, Microfluidic-based prostate cancer model for investigating the secretion of prostate-specific antigen and microRNAs in vitro
Padmyastuti A, Sarmiento MG, Dib M, Ehrhardt J, Schoon J, Somova M, Burchardt M, Roennau, Pinto PC
2023, bioRxiv, Preprint, Diseased human pancreas and liver microphysiological system for preclinical diabetes research
Rigal S, Casas B, Kanebratt KP, Wennberg Huldt C, Magnusson LU, Mullers E, Karlsson F, Clausen M, Hansson SF, Jansson Lofmark R, Ammala C, Marx U, Gennemark P, Cedersund G, Andersson TB, Vilen LK
2023, Alternatives to Laboratory Animals, OnlineFirst, Advances in Animal Models and Cutting-Edge Research in Alternatives: Proceedings of the Third International Conference on 3Rs Research and Progress, Vishakhapatnam, 2022
Naik NN, Vadloori B, Poosala S, Srivastava P, Coecke S, Smith A, Akhtar A, Roper C, Radhakrishnan S, Bhyravbhatla B, Damle M, Pulla VK, Hackethal J, Horland R, Li AP, Pati F, Singh MS, Occhetta P, Bisht R, Dandekar P, Bhagavatula K, Pajkrt D, Johnson M, Weber T, Huang J, Hysenaj L, Mallar B, Ramray B, Dixit S, Joshi S, Kulkarni M
2023, Frontiers in Pharmacology, Vol. 14, Development of a microphysiological skin-liver-thyroid Chip3 model and its application to evaluate the effects on thyroid hormones of topically applied cosmetic ingredients under consumer-relevant conditions
Tao TP, Maschmeyer I, LeCluyse EL, Rogers E, Brandmair K, Gerlach S, Przibilla J, Kern F, Genies C, Jacques C, Najjar A, Schepky A, Marx U, Kühnl J, Hewitt NJ
2022, Biomaterials and Biosystems , Vol. 7, Setup of human liver-chips integrating 3D models, microwells and a standardized microfluidic platform as proof-of-concept study to support drug evaluation
Cox B, Barton P, Class R, Coxhead H, Delatour C, Gillent E, Henshall J, Isin EM, King L, Valentin JP
2022, Journal of Extracellular Vesicles, Vol. 11, A human kidney and liver organoid-based multi-organ-on-a-chip model to study the therapeutic effects and biodistribution of mesenchymal stromal cell-derived extracellular vesicles
Nguyen VVT, Ye S, Gkouzioti V, van Wolferen ME, Yengej FY, Melkert D, Siti S, de Jong B, Besseling PJ, Spee B, van der Laan LJW, Horland R, Verhaar MC, van Balkom BWM
2022, Cells, Vol. 11, A Human Stem Cell-Derived Brain-Liver Chip for Assessing Blood-Brain-Barrier Permeation of Pharmaceutical Drugs
Koenig L, Ramme AP, Faust D, Mayer M, Flötke T, Gerhartl A, Brachner A, Neuhaus W, Appelt-Menzel A, Metzger M, Marx U, Dehne EM
2022, Pharmaceutics, Vol. 14, Proof-of-Concept Organ-on-Chip Study: Topical Cinnamaldehyde Exposure of Reconstructed Human Skin with Integrated Neopapillae Cultured under Dynamic Flow
Vahav I, Thon M, van den Broek LJ, Spiekstra SW, Ataҫ B, Lindner G, Schimek K, Marx U, Gibbs S
2022, ALTEX, A microfluidic thyroid-liver platform to assess chemical safety in humans
Kühnlenz J, Karwelat D, Steger-Hartmann T, Raschke M, Bauer S, Vural Ö, Marx U, Tinwell H, Bars R
2022, Frontiers in Toxicology, A Multi-Organ-on-Chip Approach to Investigate How Oral Exposure to Metals Can Cause Systemic Toxicity Leading to Langerhans Cell Activation in Skin
Koning JJ, Rodrigues Neves CT, Schimek K, Thon M, Spiekstra SW, Waaijman T, de Gruijl TD, Gibbs S
2021, Drug Testing and Analysis, Early view, Organ-on-a-chip: Determine feasibility of a human liver microphysiological model to assess long-term steroid metabolites in sports drug testing
Görgens C, Ramme AP, Guddat S, Schrader Y, Winter A, Dehne EM, Horland R, Thevis M
2021, Science, Vol. 373, Human microphysiological systems for drug development
Roth A, MPS-WS Berlin 2019
2021, Frontiers in Medicine, Vol. 8, An Individual Patient's "Body" on Chips – How Organismoid Theory Can Translate Into Your Personal Precision Therapy Approach
Marx U, Accastelli E, David R, Erfurth H, Koenig L, Lauster R, Ramme AP, Reinke P, Volk HD, Winter A, Dehne EM
2021, Stem Cell Research, Vol. 53, Generation of two additional integration-free iPSC lines from related human donors
Ramme AP, Faust D, Koenig L, Nguyen N, Marx U
Cell line repository/bank: Human Pluripotent Stem Cell Registry (hPSCreg)
2021, Journal of Applied Toxicology, Early view, Demonstration of the first‐pass metabolism in the skin of the hair dye, 4‐amino‐2‐hydroxytoluene, using the Chip2 skin–liver microphysiological model
Tao TP, Brandmair K, Gerlach S, Przibilla J, Géniès C, Jacques‐Jamin C, Schepky A, Marx U, J. Hewitt N, Maschmeyer I, Kühnl J
2021, Toxicology, Vol. 448, Characterization of application scenario-dependent pharmacokinetics and pharmacodynamic properties of permethrin and hyperforin in a dynamic skin and liver multi-organ-chip model
Kühnl J, Tao TP, Brandmair K, Gerlach S, Rings T, Müller-Vieira U, Przibilla J, Genies C, Jaques-Jamin C, Schepky A, Marx U, J. Hewitt N, Maschmeyer I
2020, TissUse White Paper, Multi-Organ Microphysiological Systems are Poised for Expansive Integration
2020, Scientific reports. Vol. 10, Repeated dose multi-drug testing using a microfluidic chip-based coculture of human liver and kidney proximal tubules equivalents
Lin N, Zhou X, Geng X, Drewell C, Hübner J, Li Z, Zhang Y, Xue M, Marx U, Li B
2020, In Vitro Cellular & Developmental Biology – Animal, The microfollicle: a model of the human hair follicle for in vitro studies
Ataç B, Kiss FM, Lam T, Fauler B, Edler C, Hu P, Tao TP, Jädicke M, Rütschle I, Azar RP, Youngquist S, Mielke T, Marx U, Lauster R, Lindner G, DiColandrea T
2020, International Journal of Pharmaceutics, Vol. 589, Toxicity of topically applied drugs beyond skin irritation: Static skin model vs. Two organs-on-a-chip
Tavares RSN, Tao TP, Maschmeyer I, Maria-Engler SS, Schäfer-Korting M, Winter A, Zoschke C, Lauster R, Marx U, Gaspar LR
2020, Advanced Science, Metal‐Specific Biomaterial Accumulation in Human Peri‐Implant Bone and Bone Marrow
Schoon J, Hesse B, Rakow A, Ort MJ, Lagrange A, Jacobi D, Winter A, Huesker K, Reinke S, Cotte M,Tucoulou R, Marx U, Perka C, Duda GN, Geissler S
2020, Human Reproduction, Vol. 35, A multi-organ-chip co-culture of liver and testis equivalents: a first step toward a systemic male reprotoxicity model
Baert Y, Ruetschle I, Cools W, Oehme A, Lorenz A, Marx U, Goossens E, Maschmeyer I
2020, Scientific Reports, Human multi-organ chip co-culture of bronchial lung culture and liver spheroids for substance exposure studies
Schimek K, Frentzel S, Luettich K, Bovard D, Rütschle I, Boden L, Rambo F, Erfurth H, Dehne EM, Winter A, Marx U, Hoeng J
2020, Journal of Tissue Engineering and Regenerative Medicine, Vol. 14, Reconstructed human skin shows epidermal invagination towards integrated neopapillae indicating early hair follicle formation in vitro
Vahav I, van den Broek LJ, Thon M, Monsuur HN, Spiekstra SW, Atac B, Scheper RJ, Lauster R, Lindner G, Marx U, Gibbs S
2020, ALTEX, Preprint, Biology-inspired Microphysiological systems to advance patient benefit and animal welfare in drug development
Marx U, Akabane T, Andersson T, Baker E, Beilmann M, Beken S, Brendler-Schwaab S, Cirit M, David R, Dehne EM, Durieux I, Ewart L, Fitzpatrick S, Frey O, Fuchs F, Griffith L, Hamilton G, Hartung T, Hoeng J, Hogberg H, Hughes D, Ingber D, Iskandar A, Kanamori T, Kojima H, Kuehnl J, Leist M, Li B, Loskill P, Mendrick D, Neumann T, Pallocca G, Rusyn I, Smirnova L, Steger-Hartmann T, Tagle D, Tonevitsky A, Tsyb S, Trapecar M, van de Water B, van den Eijnden-van Raaij J, Vulto P, Watanabe K, Wolf A, Zhou X, Roth A
2020, Current Opinion in Toxicology, Journal pre-proof, The universal physiological template – a system to advance medicines
Dehne EM, Marx U
2020, Elsevier, 441-462, Automation and opportunities for industry scale-up of microphysiological systems in: Organ-on-a-Chip: Engineered Microenvironments for Safety and Efficacy Testing
Dehne EM, Erfurth H, Muhsmann AK, Marx U
2020, Elsevier, 429-439, Human body-on-a-chip systems in: Organ-on-a-Chip: Engineered Microenvironments for Safety and Efficacy Testing
Dehne EM, Marx U
2019, Stem Cell Research, Vol. 41, Generation of four integration-free iPSC lines from related human donors
Ramme AP, Faust D, Koenig L, Marx U
Cell line repository/bank: Human Pluripotent Stem Cell Registry (hPSCreg)
2019, Current Opinion in Toxicology, Vol. 17, Microphysiological systems in the evaluation of hematotoxicities during drug development
Dehne EM, Winter A, Marx U
2019, Future Science OA, Vol. 5, No. 8, Autologous induced pluripotent stem cell-derived four-organ-chip
Ramme AP, Koenig L, Hasenberg T, Schwenk C, Magauer C, Faust D, Lorenz AK, Krebs AC, Drewell C, Schirrmann K, Vladetic A, Lin GC, Pabinger S, Neuhaus W, Bois F, Lauster R, Marx U, Dehne EM
2019, Elsevier, 279-284, Biologically-Inspired Microphysiological Systems in: The History of Alternative Test Methods in Toxicology
Dehne EM, Hickman J & Shuler M
2018, ALTEX, Optimizing drug discovery by Investigative Toxicology: Current and future trends
Beilmann M, Boonen H, Czich A, Dear G, Hewitt P, Mow T, Newham P, Oinonen T, Pognan F, Roth A, Valentin JP, Van Goethem F, Weaver RJ, Birk B, Boyer S, Caloni F, Chen AE, Corvi R, Cronin MTD, Daneshian M, Ewart LC, Fitzgerald RE, Hamilton GA, Hartung T,Kangas JD, Kramer NI, Leist M, Marx U, Polak S, Rovida C, Testai E, Van der Water B, Vulto P, Steger-Hartmann T
2018, Nature Scientific Reports, Simultaneous evaluation of anti-EGFR-induced tumour and adverse skin effects in a microfluidic human 3D co-culture model
Hübner J, Raschke M, Rütschle I, Gräßle S, Hasenberg T, Schirrmann K, Lorenz A, Schnurre S, Lauster R, Maschmeyer I, Steger-Hartmann T, Marx U
2018, Bioengineering 2018, Bioengineering of a Full-Thickness Skin Equivalent in a 96-Well Insert Format for Substance Permeation Studies and Organ-on-a-Chip Applications
Schimek K, Hsu HH, Boehme M, Kornet JJ, Marx U, Lauster R, Pörtner R, Lindner G
2018, J Vis Exp., A Method for Determination and Simulation of Permeability and Diffusion in a 3D Tissue Model in a Membrane Insert System for Multi-well Plates
Hsu HH, Kracht JK, Harder LE, Rudnik K, Lindner G, Schimek K, Marx U, Pörtner R
2018, Stem Cell Res Ther., The role of fibrinolysis inhibition in engineered vascular networks derived from endothelial cells and adipose-derived stem cells
Mühleder S, Pill K, Schaupper M, Labuda K, Priglinger E, Hofbauer P, Charwat V, Marx U, Redl H, Holnthoner W
北京佰司特科技有限责任公司 (https://www.best-sciences.com)
类器官串联芯片培养仪-HUMIMIC;灌流式细胞组织类器官代谢分析仪-IMOLA;光片显微镜-LSM-200;
蛋白质稳定性分析仪-PSA-16;单分子质量光度计-TwoMP;超高速视频级原子力显微镜-HS-AFM;
全自动半导体式细胞计数仪-SOL COUNT;农药残留定量检测仪—BST-100;台式原子力显微镜-ACST-AFM;微纳加工点印仪-NLP2000DPN5000;






